Thermal enhancement of the oxidation layer of micron-sized aluminum powder and its anti-oxidation performance
Published 27-01-2024
Keywords
- micron-sized aluminum powder,
- core-shell structure,
- oxidation layer enhancement,
- thermal response
Copyright (c) 2024 Cambridge Science Advance

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
How to Cite
Abstract
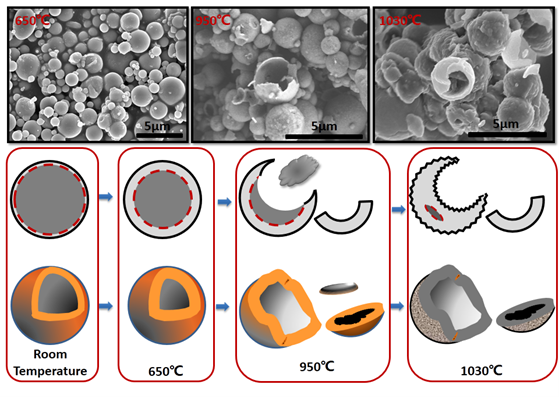
Micron-sized aluminum powder, with its typical core-shell structure, exhibits oxidation behavior upon slow heating in an oxidative atmosphere that is related to the particle size of the aluminum powder. A particle size-dependent thermal response mechanism for micron-sized aluminum powder is established: a "shell-breaking eruption" thermal response mechanism. The oxide layer is thermally enhanced, and the anti-oxidation reaction behavior mechanism of the enhanced samples is studied. Aluminum powder samples, heated slowly to specific temperatures to obtain changes in the oxide layer structure, are analyzed using a thermal analyzer to compare the slow heating response behavior of aluminum powder before and after the oxidation layer changes. It was found that after thermal enhancement of the aluminum powder oxidation layer, which changed from amorphous to γ-phase and whose thickness increased to twice the original thickness, the slow oxidation process of micron-sized aluminum powder was inhibited, and the aluminum powder was completely deactivated in the slow heating response.
References
- Dreizin E L, Schoenitz M. Correlating ignition mechanisms of aluminum-based reactive materials with thermoanalytical measurements. Progress in Energy & Combustion Science, 2015, 50:81-105.
- Starik A M, Savel’Ev A M, Titova N S. Specific features of ignition and combustion of composite fuels containing aluminum nanoparticles (Review). Combustion, Explosion, and Shock Waves, 2015, 51(2):197-222.
- Sundaram D S, Yang V, Zarko V E. Combustion of nano aluminum particles (Review). Combustion, Explosion, and Shock Waves, 2015, 51(2):173-196.
- Maggi F, Dossi S, Paravan C, et al. Activated aluminum powders for space propulsion. Powder Technology, 2015, 270:46-52.
- Li Ying, Song Wulin, Xie Changsheng, et al. Progress in the Application of Nano Aluminum Powder in Solid Propellants. ACTA ARMAMENTARII, 2005, 26(1):121-125.
- An Ting, Zhao Fengqi, Yi Jianhua, et al. Preparation, Characterization, Decomposition Mechanism and Non-Isothermal Decomposition Reaction Kinetics of the Super Thermite Al/CuO Precursor. ACTA PHYSICO-CHIMICA SINICA, 2011, 27(2): 281-288.
- Zhu Yan-Li, Jiao Qing-Jie, Huang Hao, et al. Effect of Aluminum Particle Size on Thermal Decomposition of AP. CHEMICAL JOURNAL OF CHINESE UNIVERSITIES, 2013, 34(3): 662-667.
- Zeng Liang, Jiao Qing-jie, Ren Hui, Zhou Qing. Effect of Particle Size of Nano-aluminum Powder on Oxide Film Thickness and Active Aluminum Content. Chinese Journal of Explosives & Propellants, 2011(4):26-29.
- Li Xin, Zhao Fengqi, Hao Haixia, et al. Research on Ignition and Combustion Properties of Different Micro/nano-aluminum Powders. Acta Armamentarii, 2014, 35(5):640-647.
- Puri P. Multi scale modeling of ignition and combustion of micro and nano aluminum particles. The Pennsylvania State University: Dissertations & Theses - Gradworks, 2008.
- Zhu X, Schoenitz M, Dreizin E L. Aluminum Powder Oxidation in CO2 and Mixed CO2/O2 Environments. Journal of Physical Chemistry C, 2009, 113(16):6768-6773.
- Badiola C, Gill R J, Dreizin E L. Combustion characteristics of micron-sized aluminum particles in oxygenated environments. Combustion & Flame, 2011, 158(10):2064-2070.
- Sippel T R, Pourpoint T L, Son S F. Combustion of Nanoaluminum and Water Propellants: Effect of Equivalence Ratio and Safety/Aging Characterization. Propellants Explosives Pyrotechnics, 2013, 38(1):56–66.
- Risha G A, Sabourin J L, Yang V, et al. Combustion and Conversion Efficiency of Nanoaluminum-Water Mixtures. Combustion Science and Technology, 2007, 180(12):2127-2142.
- Rossi S, Dreizin E L, Law C K. Combustion of Aluminum Particles in Carbon Dioxide. Combustion Science and Technology, 2001, 164(1):209-237.
- Ernst L F, Dryer F L, Yetter R A, et al. Aluminum droplet combustion in fluorine and mixed oxygen/fluorine containing environments. Proceedings of the Combustion Institute, 2000, 28(1):871–878.
- Gan Y, Li Q. Combustion characteristics of fuel droplets with addition of nano and micron-sized aluminum particles. Combustion & Flame, 2011, 158(2):354-368.
- Kong C, Dan Y, Qiang Y, et al. Morphological changes of nano-Al agglomerates during reaction and its effect on combustion. Combustion & Flame, 2016, 165:11-20.
- He Lirong, Xiao Leqin, Ding Haiqin, et al. TG-DSC research of shell’s effect on activity of nano-aluminum powder. Ordnance Material Science and Engineering, 2013, 36(5): 21-25.
- Steenkiste T H V, Smith J R, Teets R E. Aluminum coatings via kinetic spray with relatively large powder particles. Surface & Coatings Technology, 2002, 154(2–3):237-252.
- Cheng Zhipeng, Yang Yi, Wang Yi, et al. Oxidation Ability of Nanocrystalline Ni-Coated Al Powders. ACTA PHYSICO-CHIMICA SINICA, 2008, 24(1):152-156.
- Zeng Liang. Core-shell Structure Aluminum Powder Impact on the Reaction Process of Aluminized Explosive. BEIJING: BEIJING INSTITUTE OF TECHNOLOGY, 2011:19-33.
- Zeng Liang, Jiao Qingjie, Ren Hui, et al. Studies on Oxide Film Thickness and Activity of Micron Aluminum Powder. Transactions of Beijing Institute of Technology, 2012, 32(2): 206-211.
- Rufino B, Boulc’H F, Coulet M V, et al. Influence of particles size on thermal properties of aluminium powder. Acta Materialia, 2007, 55(8):2815-2827.
