Published 12-11-2024
Keywords
- Magnesium Isoglycyrrhizinate,
- Glutathione,
- Traditional Chinese Medicine Compatibility,
- Drug Stability
Copyright (c) 2024 Cambridge Science Advance

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
How to Cite
Abstract
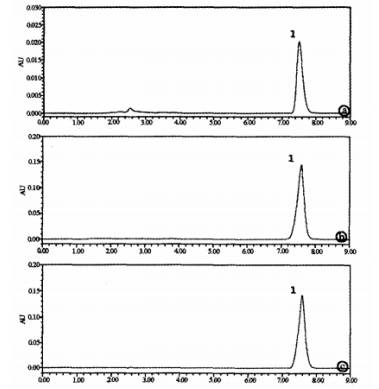
Objective: To investigate the stability of magnesium isoglycyrrhizinate (Mg IG) injection solution when mixed with reduced glutathione (GSH) injection solution at room temperature. Method: High-performance liquid chromatography was used to determine the concentration changes of Mg IG injection solution mixed with GSH injection solution in 5% and 10% glucose solutions. Results: No significant changes in concentration were observed within 0, 0.5, 1, 1.5, 2, and 4 hours after mixing the Mg IG injection solution with the GSH injection solution. Conclusion: Mg IG injection solution can be mixed with GSH injection solution in 5% and 10% glucose solutions and should be used within 4 hours.
References
- WANG P, WU XM. Therapeutic effect of magnesium isoglycyrrhizinate on carbon tetrachloride -induced chronic liver injury in rats[J]. Chin J New Drugs Clin Remed,2004,23(12):833-836. (in Chinese)
- OU MH. Advances in pharmacological and clinical research on magnesium isoglycyrrhizinate[J]. China Pharm,2010, 19(15):83-85. (in Chinese)
- YUAN PG, ZHANG DZ. Mechanism of action and clinical application of reduced glutathione[J]. Drug Eval,2006,3(5): 385-390. (in Chinese)
- HE M, ZHAN M, LIU RM, et al. Systematic review on reduced glutathione in the treatment of drug-induced liver disease[J]. China Pharm,2010,21(32):3049-3052. (in Chinese)
- DAI JH, IWATANIY, ISHIDA T, et al. Glycyrrhizin enhances interleukin-12 production in peritoneal macrophages[J]. Immunology,2001,103(2):235-243.
- ABE M, AKBAR F, HASEBE A, et al. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis[J]. J Gastroenterol,2003,38(10):962-967.
- ZHANG Q, WU P, ZHANG JJ. The protective effect of the 18-α isomer of Glycyrrhizic Acid inhuman hepatocytes with chemical injury[J]. The Chinese Journal of Clinical Pharmacology, 2011, 16(12):1357-1360. (in Chinese)
- CHEN WH, LU LG, ZENG MD, et al. Effect of magnesium isoglycyrhizinate on the proliferation and oxidative stress of rat hepatic stellate cells in vitro[J]. Chin J Hepatol,2006, 14(6):426-430. (in Chinese)
- ZHONG CM, LI ZL, CHEN ZF. Clinical application of reduced glutathione[J]. P Clin Med,2010,19(5A):325-328. (in Chinese)
- WANG YX, ZHANG XQ, WAN SZ. Compatible stability of magnesium isoglycyrrhizinate in 6 mixtures[J]. Chin J Hosp Pharm 2008,28(20):1801-1803. (in Chinese)
- ZHOU XQ, FANG J. Stability of reduced glutathione sodium in four different injections[J]. China Pharm,2008,19(35): 2759-2761. (in Chinese)
